Launch of a Joint EADO–EUMelaReg ctDNA Project
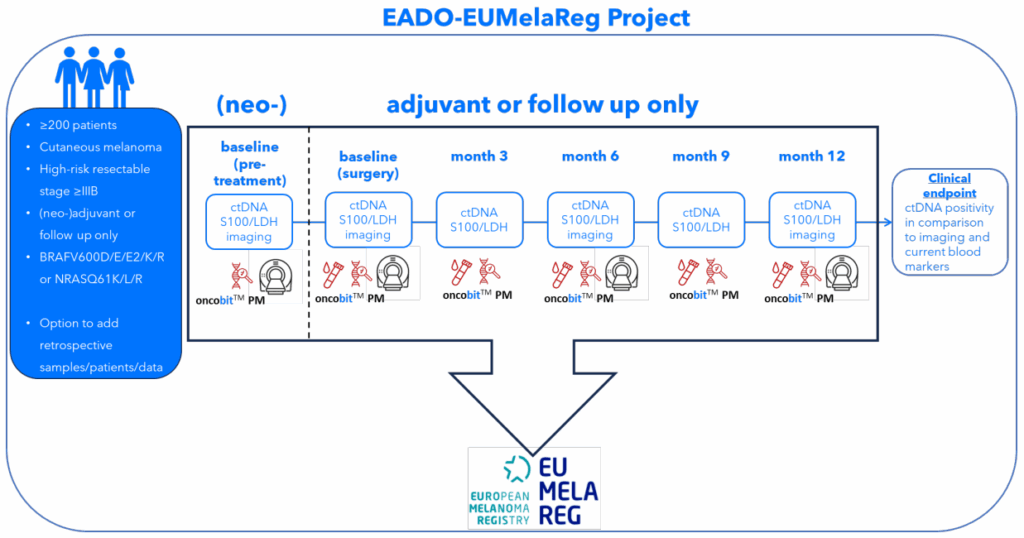
We are pleased to announce the launch of a joint EADO–EUMelaReg project aimed at integrating circulating tumor DNA (ctDNA) into routine melanoma follow-up. The project will evaluate the clinical utility of ctDNA assessed using Oncobit’s CE-IVDR–marked digital PCR solution to detect relapse and to complement or potentially replace established monitoring approaches. By closely reflecting real-world clinical practice and leveraging the strengths of the EUMelaReg registry, this initiative seeks to advance precision monitoring in melanoma care.